
Rare Diseases
In depth articles on rare diseases, rare disease research, solutions and treatments

Chris Moore from Veeva Europe explores how companies are overcoming challenges to develop, launch and educate on new rare disease medicines faster

Dana Vigier from Alexion considers the challenges facing rare disease treatment and the importance of patient-centricity to improve outcomes

Dan Williams from SynaptixBio considers the problems facing the ongoing treatment of rare diseases, as well as how the pharma industry is moving forwards in their treatment

Elizabeth Sachnoff from Propharma considers the landscape of clinical trials for rare diseases, and how these could be improved

Nazim Kanji and Huw Jones from Quotient Sciences consider orphan drug development and the benefit of CRO/CDMO collaboration, specifically in terms of paediatric rare diseases

The rare disease treatment market has been rapidly changing and receive deserved recognition

Patient advocacy organisations can form an effective partnership between clinical trials and patients for more efficient trials
Pharmafile speaks to Chiesi Group: Chiesi Group tells Pharmafile about the challenges facing rare disease research and their hopes for the future of this area

Aaron Bean at Veeva explores the challenges of rare diseases: With up to 30 million people in EU living with rare diseases, Veeva discusses the challenges facing these rare diseases

Many advancements have been made within the rare disease field, including gene editing and viral delivery strategies
Takeda tells Pharmafile about the role of combination treatments in rare diseases, and strategies to make them more accessible
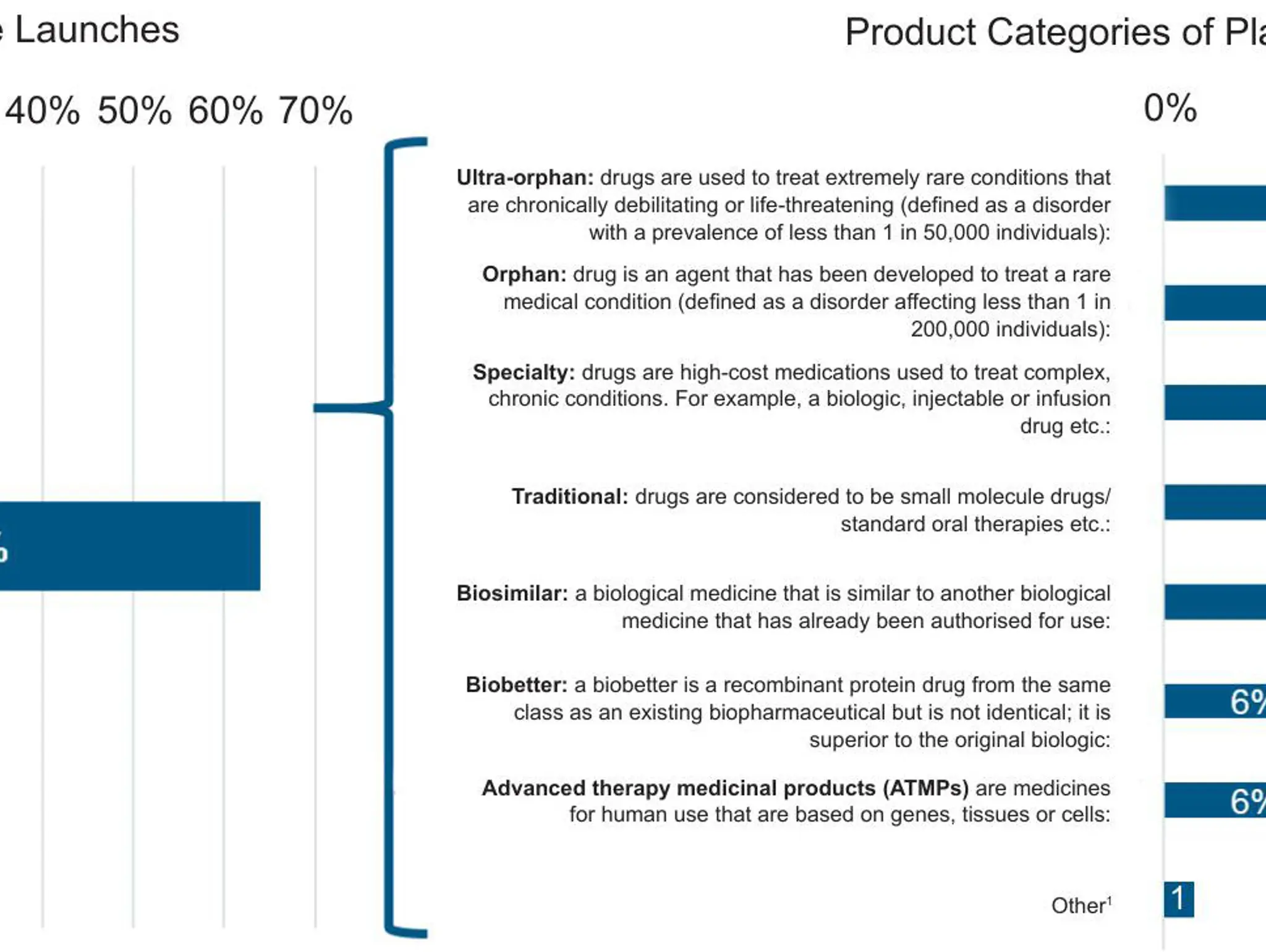
Rare and ultra-rare diseases impact a surprising amount of people, this article explores the field of rare diseases as well as the diagnostic journey many patients take