
News
News articles from Pharmafile - therapy areas in focus

Don't miss the biggest upcoming events in the world of pharma.
ANGLE has announced that it has signed a supplier agreement with AstraZeneca for the development and validation of a methodology that will leverage angle’s existing DNA damage response (DDR) assay for the detection of micronuclei in circulating tumour cells (CTCs) as a measure of DDR.
GSK has announced that the FDA has accepted for review a biologics license application (BLA) for its 5-in-1 meningococcal abcwy (menabcwy) vaccine candidate.

Regeneron Pharmaceuticals and Mammoth Biosciences have announced a collaboration for the research, development and commercialisation of in vivo CRISPR-based gene-editing therapies for multiple tissue and cell types.
Eko Health has announced that the FDA has approved its low ejection fraction (low EF) detection artificial intelligence (AI).

Pfizer has announced that the FDA has approved Beqvez (fidanacogene elaparvovec-dzkt) for the treatment of adults with moderate-to-severe haemophilia b.
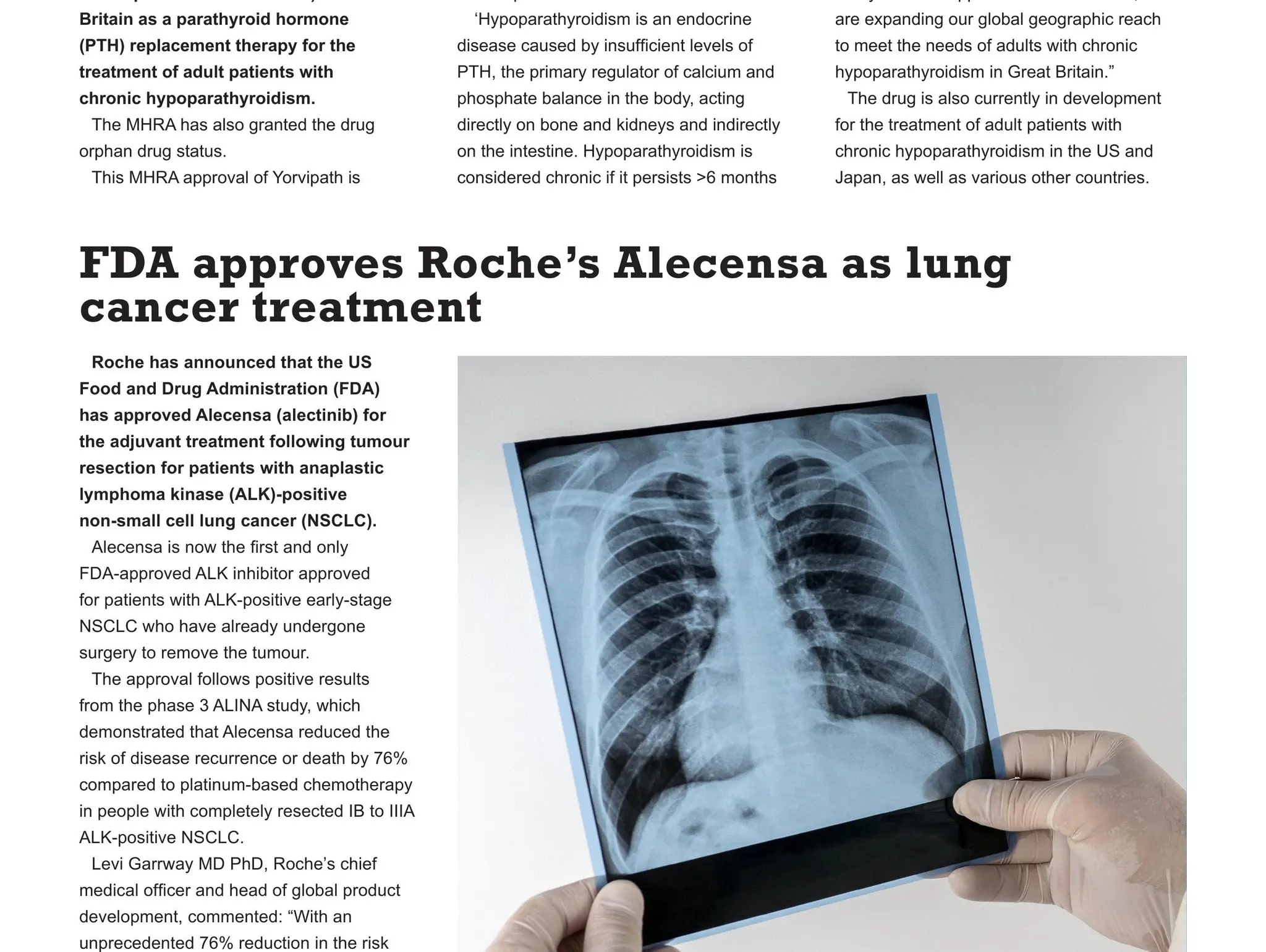
Roche has announced that the FDA has approved Alecensa (alectinib) for the adjuvant treatment following tumour resection for patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC).
Ascendis Pharma has announced that the MHRA has granted marketing authorisation for yorvipath (palopegteriparatide; developed as transcon pth) in GB as a parathyroid hormone (pth) replacement therapy for the treatment of adult patients with chronic hypoparathyroidism.
Janssen, a J&J company, has announced that the CHMP of the EMA has recommended Rybrevant (amivantamab) in combination with chemotherapy.

GSK and Save the Children have announced that they will be renewing their partnership for an additional five years.

Astellas pharma has announced that it is preparing to submit a planning application to build a new state-of-the-art facility at an estimated cost of €330m in tralee, county kerry, ireland.

Pharmanovia has announced that it will expand its neurology portfolio with the acquisition of a selection of central nervous system (CNS) brands from Sanofi.